Envis Centre, Ministry of Environment & Forest, Govt. of India
Printed Date: Friday, August 29, 2025
More About Japanese Encephalitis
Introduction
Japanese encephalitis (JE) is a common mosquito-borne viral encephalitis found in Asia. It occurs mainly in the rural and agricultural areas. Over 50,000 cases are reported annually from Southeast Asia, India, China, Japan and Korea. China contributes more than 50% and India contributes another 20%. The virus was isolated for the first time in the world from a post-mortem human brain in Japan in 1933.
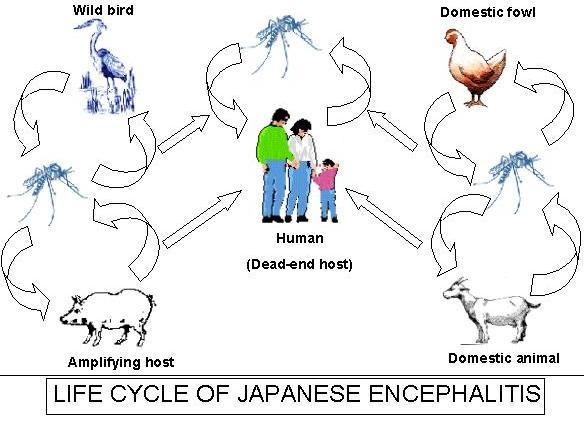
JE virus is transmitted chiefly by the bites of mosquitoes in the Culex vishnui complex; the vector species depends on the specific geographic area. In Asia, C. tritaeniorhynchus is the principal vector. This species has a wide host range, including domestic animals, birds, and humans. Viral infection rates in the mosquitoes range from less than 1% to 3%. The virus is transmitted in an enzootic cycle among mosquitoes and vertebrate-amplifying hosts, chiefly domestic pigs, Ardeid (wading) birds and Swine function as viremic amplifying hosts in the transmission cycle.
In areas where JE is endemic, annual incidence ranges from 1 to 10 per 10,000. Children less than 15 years of age are principally affected. In developed countries of Asia and in areas, where children are protected by immunization, a secondary increase in JE incidence has been observed in the elderly.
Indian scenario
JE was clinically diagnosed for the first time in 1955 at Vellore, erstwhile North Arcot district of Tamil Nadu. The first major outbreak of JE occurred in 1973 in two districts (Burdwan, Bankura) of West Bengal with 700 cases and 300 causalities followed by another outbreak in 1976. Since then, the number of outbreaks have been reported from the states of Bihar, Uttar Pradesh, Assam, Manipur, Andhra Pradesh, Karnataka, Madhya Pradesh, Maharashtra, Tamil Nadu, Haryana, Kerala, West Bengal, Orissa and Union Territories of Goa and Pondicherry.
Transmission
Transmission is usually seasonal, following the prevalence of mosquitoes. Epidemics usually occur towards the end of the wet season. Patterns of JE viral transmission vary regionally, within individual countries and from year to year. In temperate zones of China, Japan, Korea and northern areas of Southeast Asia, Japanese encephalitis is transmitted during summer and early autumn from May to October. In North India and Nepal, transmission occurs from June to November, and in south India and Sri Lanka, epidemics are found from September to January.
Burden
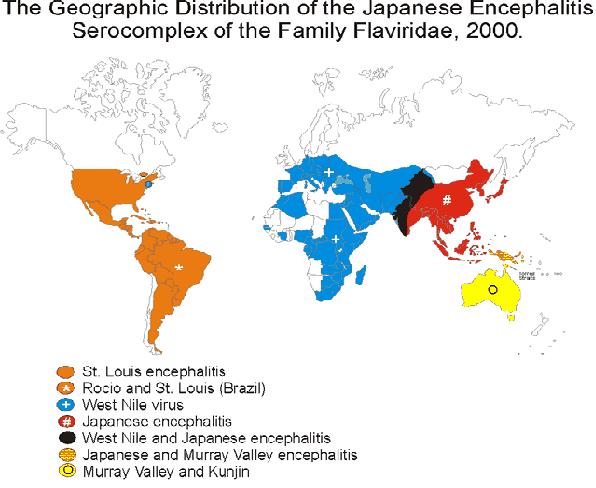
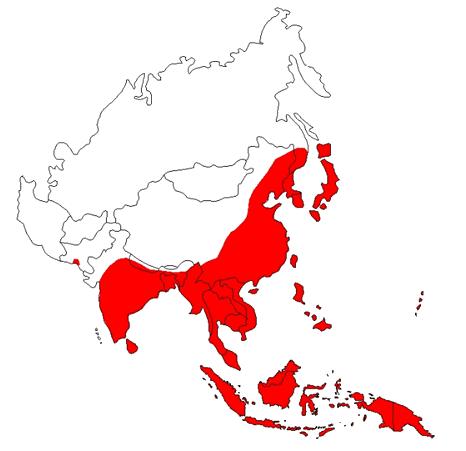
- Distribution of JE in Asia
.JPG)
Clinical Symptons
- Incubation period is 4-14 days. The case progresses through four stages.
- Prodromal illness stage (2-3 days)
- Acute stage (3-4 days)
- Subacute stage (7-10 days)
- Convalescence stage (4-7 weeks)
Prodromal illness (2-3 days) - Onset may be characterized by abrupt headache, respiratory symptoms, anorexia, nausea, abdominal pain, vomiting and sensory changes including psychotic episodes. A low - grade fever or minor respiratory symptoms may be the only clinical expression of JE.
The acute stage (3-4 days) - It is heralded by a high fever. Convulsions, confusion, disorientation, delirium or somnolence progresses to coma. There may be oliguria, diarrhoea and relative bradycardia. Fatal cases usually progress rapidly to coma and the patient dies within 10 days.
Subacute stage (7-10 days) - The severity of the central nervous system lessens but pneumonia, urinary tract infections or bedsores may be management problems and in some instances, are life threatening.
Convalescence stage (4-7 weeks) - It is prolonged with weakness, lethargy, incoordination, tremors and neuroses. Weight loss may be severe.
Most infections are asymptomatic but if clinical illness develops, the case-fatality rate can be as high as 30%. Neuropsychiatric sequelae are reported in 50% of survivors. In endemic areas, children are at the greatest risk of infection; however, multiple factors such as occupation, recreational exposure, gender (possibly reflecting exposure), previous vaccination and naturally acquired immunity alter the potential for infection and illness.
Infections in pregnant women during the first and second trimester have been associated with miscarriages. There is no specific drug to treat Japanese encephalitis. A formal inactivated vaccine prepared in mice is used widely in Japan, China, India, Korea, Taiwan and Thailand. This vaccine is currently available for human use in the United States, for individuals who might be traveling to endemic countries.
Mortality/Morbidity
Only 1 per 250 infections results in symptomatic disease. Mortality rates in places with intensive care capabilities are 5-10%. In less developed areas, mortality rates may exceed 35%. Worldwide, more than 10,000 reported deaths occur per year.
Approximately, 33-50% of patients with symptomatic disease who survive have major neurologic sequelae at 1 year, including seizure disorders, motor or cranial nerve paresis or movement disorders.
- Nearly 75% of symptomatic patients with JEV who are evaluated 5 years after the disease score lower than uninfected subjects on standardized tests.
- The risk factors for death include demonstration of virus in the cerebral spinal fluid (CSF), low levels of immunoglobulin G (IgG)/immunoglobulin M (IgM) in CSF or serum and a decreased sensorium.
Diagnosis
The etiological diagnosis of JE is mainly based on serological testing, using IgM-capture ELISA that detects specific IgM in the cerebrospinal fluid or in the blood of almost all patients within four to seven days of disease onset. Other diagnostic methods include recently developed dot-blot or immuno-precipitation IgM assays, suitable for use in the field and traditional tests that monitor significant changes of JE-specific antibody titres in sequential serum samples. The virus may be recovered in various cell cultures inoculated with blood collected during the early stages of the disease or from the cerebrospinal fluid (or brain) in advanced cases of encephalitis. Polymerase chain reaction-based tests for the detection of virus-specific genomic material, particularly in cerebrospinal fluid, have also been developed.
Vaccination
In infants and children whose primary immunization series included 0.5-mL doses, a 1.0-mL booster dose (0.5 mL for children <3 years of age) may be administered 2 years after the primary series.
| Doses |
Subcutaneous route |
Comments |
| |
1–2 years of age |
3 or more years of age |
|
| Primary series 1, 2, and 3 |
0.5 mL |
1.0 mL |
Days 0, 7, and 30 |
| Booster* |
0.5 mL |
1.0 mL |
1 dose at 24 months or later |
In vaccinees who have completed a three-dose primary series, the full duration of protection is unknown; therefore, definitive recommendations cannot be made.
Source: CDC.
Click here for Bibliography on Japanese encephalitis